________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
Aimovig® is a potent and selective CGRP receptor antagonist being studied in both episodic and chronic migraine patients.1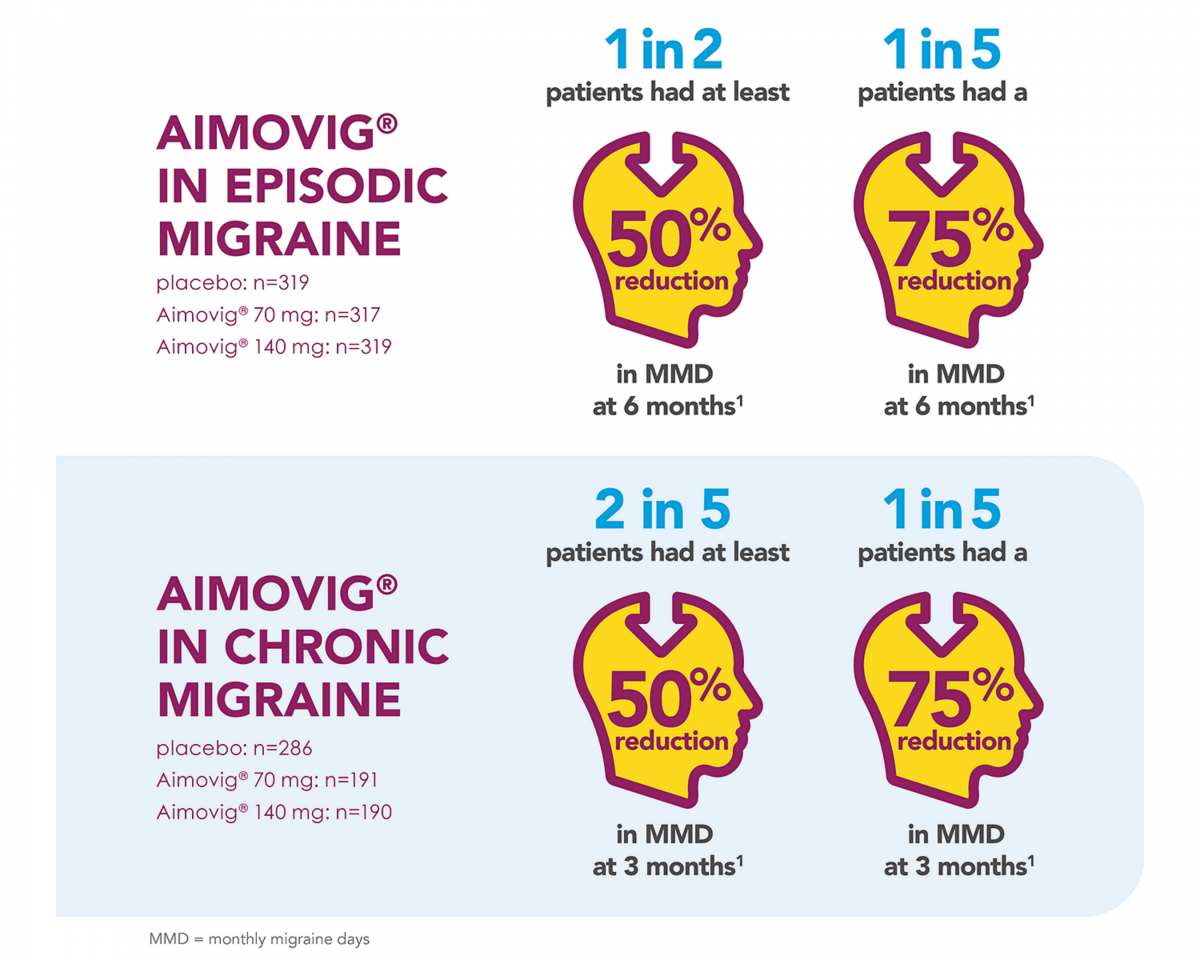
Expand each section below to learn more about the Aimovig® efficacy data across the migraine spectrum.
Reference
- Aimovig SmPC available at www.medicines.ie [accessed July 2021]
Efficacy of Aimovig® in Episodic Migraine Patients
- In a phase 3 pivotal study of episodic migraine patients, 50% of patients achieved a ≥50% reduction in mean monthly migraine days (MMD) from baseline with Aimovig 140mg.1,2
- Overall 43% of patients receiving 70mg achieved a ≥50% reduction in mean monthly migraine days from baseline.1,2
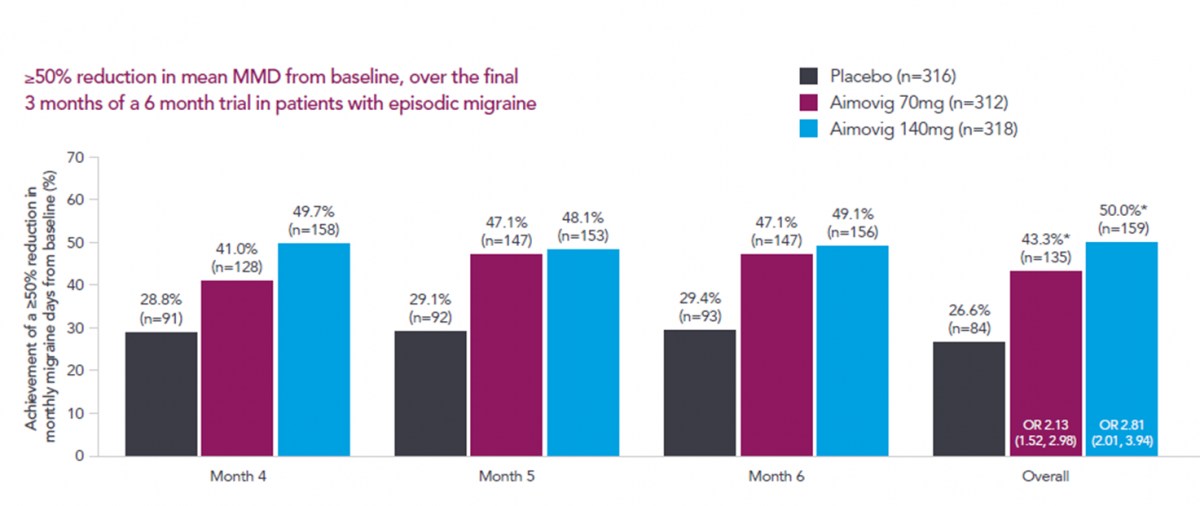
These patients achieved a reduction of 3.2 and 3.7 mean monthly migraine days at 6 months, for the 70mg and 140mg respectively.2
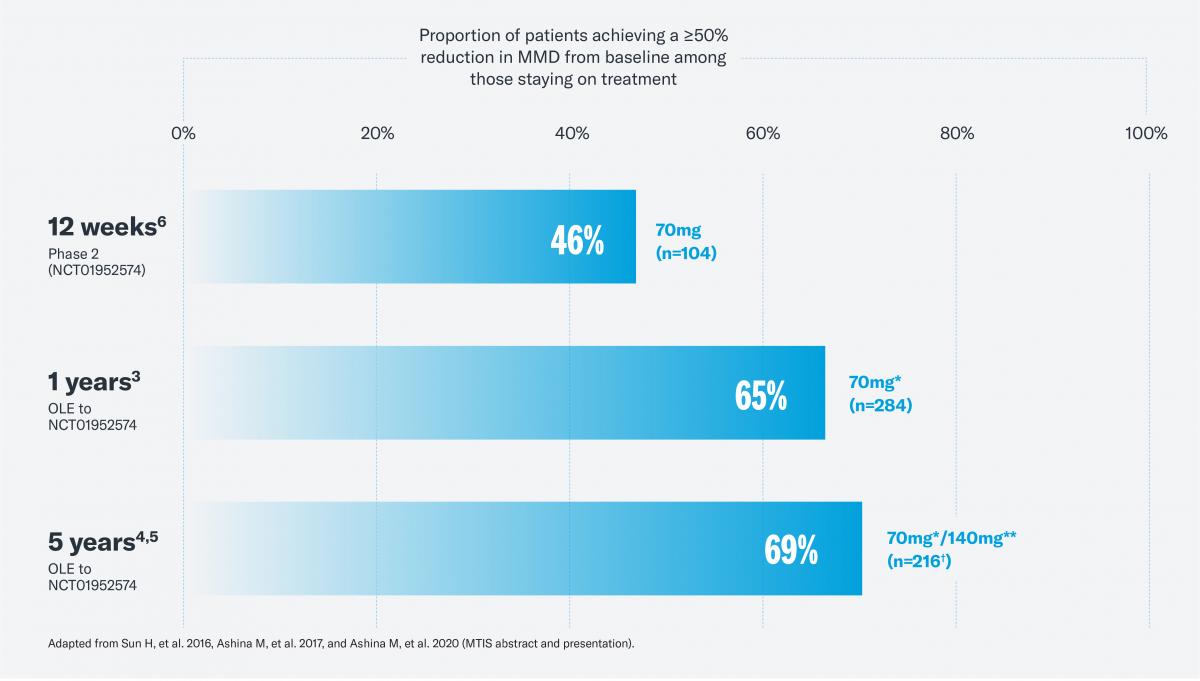
Aimovig® has been evaluated in a 5-year long-term extension study.5 The data suggests that benefits with Aimovig® can improve over time among responders.3-5 Of the patients that continued treatment with Aimovig over a 5-year period, 71% achieved a ≥50% reduction, 47.1% achieved a ≥75% reduction and 35.5% achieved a 100% reduction in MMD during the last 4 weeks of treatment compared to baseline.5
Reference
- Aimovig SmPC available at www.medicines.ie [accessed July 2021]
- Goadsby PJ, et al. N Engl J Med 2017; 377: 2123-2132.
- Ashina M, et al. Neurology 2017; 89(12): 1237-1243.
- Ashina M, et al. Headache 2019; 59 (Suppl 1): 25.
- Ashina M, et al. Eur J Neurol. 2021 28(5):1716-1725
- Sun H, et al. Lancet Neurol 2016; 15: 382-390.
Efficacy of Aimovig® in Chronic Migraine Patients
Aimovig® has also been shown to reduce monthly migraine days for chronic migraine patients.1,2
During a phase 2 pivotal study, patients receiving Aimovig 70 mg and 140 mg experienced significant reductions in MMD of 6.6 days from baseline at week 12 compared to a 4.2 day reduction in the placebo arm.2
For the sub-group of patients with a response at a 100% threshold, an even greater reduction in MMD was observed than in the overall Aimovig® treated population. This sub-group analysis shows that patients who respond to treatment with Aimovig® can achieve a reduction of up to 15 monthly migraine days compared to the overall treated population.3
This reduction was sustained for up to 1 year in the open-label extension of the study.4 Pooled across the two doses, patients achieved a mean reduction of 9.3 monthly migraine days.4
Dashed lines indicate transition from end of parent study to Week 4 of OLTP. *Patients completing 52 weeks of IP. Of the 609 patients enrolled in the OLTP phase, 469 patients completed 52 weeks of IP (70mg N=266, 140mg N=203). Of these patients, 379 patients (70mg n=214, 140mg n=165) were available for analysis at the corresponding visit at Week 52. Baseline mean MMD for DTBP: placebo 18.2, Aimovig® 70mg 17.9, Aimovig® 140mg 17.8.3 Baseline mean MMD for OLTP: 18.1. CI, confidence interval; DBTP, double-blind treatment phase; IP, investigational product; MMD, monthly migraine days; OLTP, open-label treatment phase.
Aimovig has also been shown to reduce monthly migraine days in patients who have failed ≥3 prior prophylactic treatments.5
In a subgroup analysis of patients in the open label treatment phase, Aimovig® led to a sustained reduction in monthly migraine days in this difficult to treat patient cohort.5 Almost one in two chronic migraine patients with ≥3 prior treatment failures achieved a ≥50% reduction in MMD with Aimovig® at 52 weeks.5
References
- Aimovig SmPC available at www.medicines.ie [accessed July 2021]
- Tepper S, et al. Lancet Neurol 2017; 16: 425-434.
- Brandes JL, et al. Cephalalgia 2019. DOI: 10.1177/0333102419894559.
- Tepper SJ, et al. Poster PF115LB. AHS, San Francisco; June 28-July 1, 2018.
- Weatherall, M. et al. Poster IHC-PO-175. IHC, Dublin, Ireland, 5–8 September, 2019



