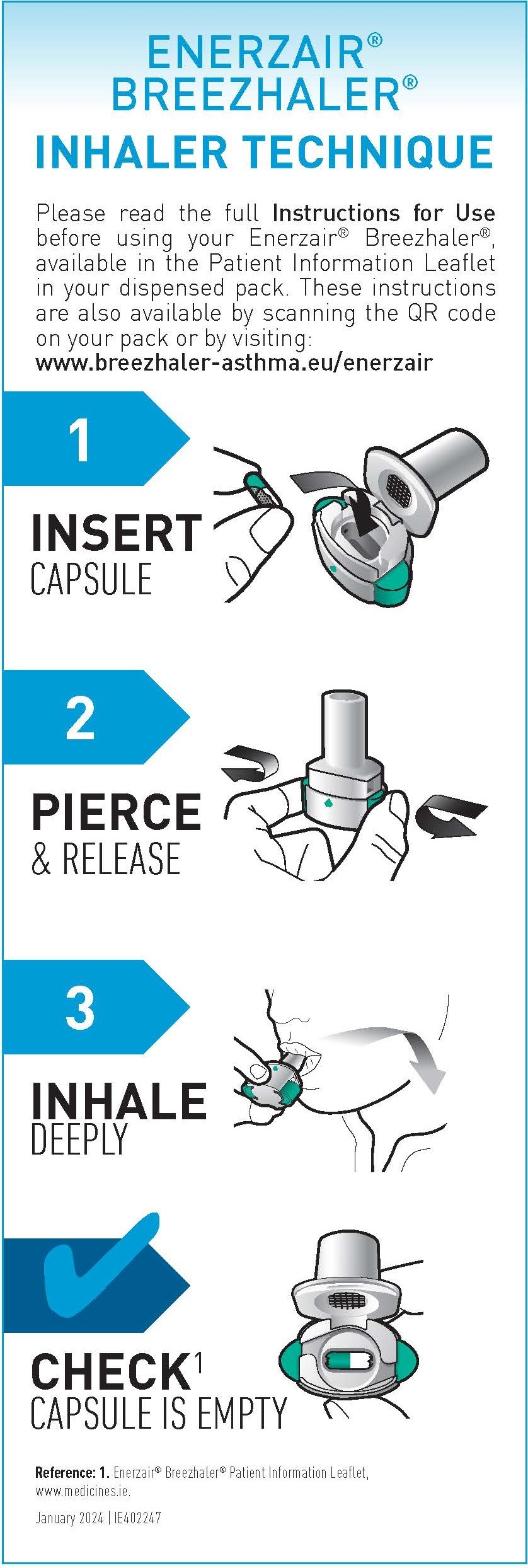
This website is intended for healthcare professionals registered within the Republic of Ireland. By entering this website, you are confirming that you are a healthcare professional registered within Republic of Ireland. This website may contain professional resources, therapy area materials and promotional information about Novartis products.
FA-11211732 | June 2024