________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
Inclisiran is indicated in adults with primary hypercholesterolaemia (heterozygous familial and non-familial) or mixed dyslipidaemia, as an adjunct to diet:
- in combination with a statin or statin with other lipid-lowering therapies in patients unable to reach LDL-C goals with the maximum tolerated dose of a statin, or
- alone or in combination with other lipid-lowering therapies in patients who are statin-intolerant, or for whom a statin is contraindicated.2
Inclisiran was generally well-tolerated, with a safety profile similar to placebo apart from injection-site reactions1
Injection-site adverse reactions were more frequent with inclisiran than placebo, with between-group differences of 1.7% in ORION-10 and 4.2% in ORION-11; the majority of these reactions were mild, with none being severe or persistent.1
Discontinuation rates due to AEs were balanced among both treatment groups: 2.4% (n=19) vs 2.2% (n=17) in ORION-10 and 2.8% (n=23) vs 2.2% (n=18) in ORION-11, of patients treated with inclisiran and placebo, respectively.1
No clinically significant interactions with other medicinal products are expected with inclisiran.2
Laboratory results with respect to liver and kidney function, levels of creatine kinase and high-sensitivity C-reactive protein, and platelet count were also similar in the inclisiran and placebo groups in each trial.1
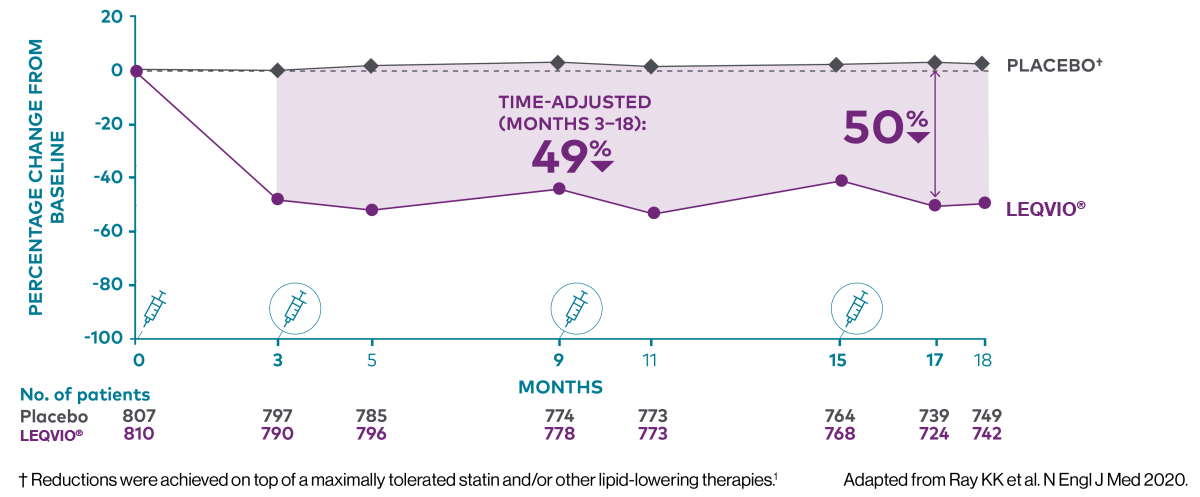
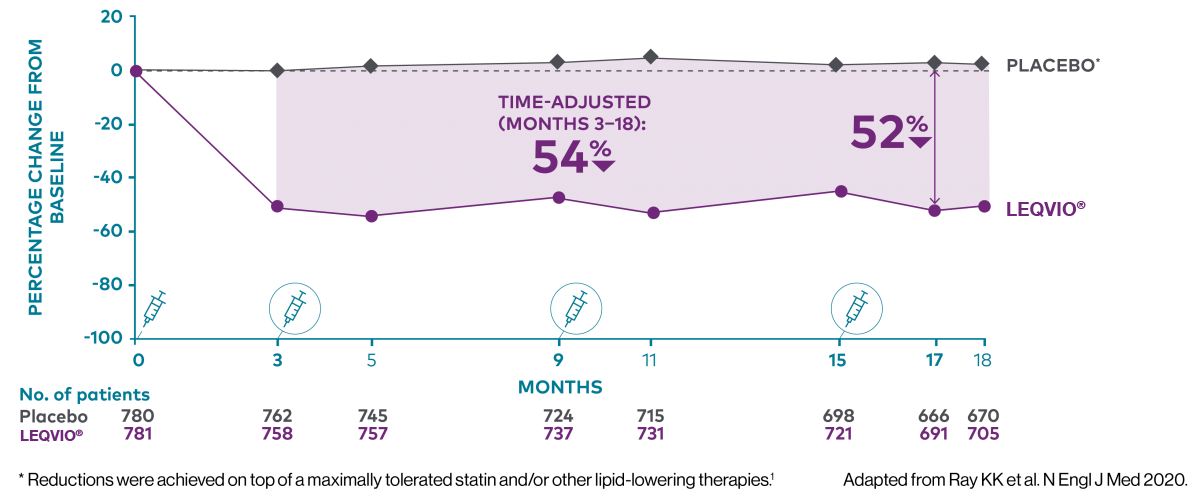
Two maintenance doses a year‡ to lower LDL-C all year long1,2
Learn more about the dosing and administration of LEQVIO®.
The effect of LEQVIO® on cardiovascular morbidity and mortality has not yet been determined.2
LEQVIO® is not reimbursed in Ireland at this time.
* ASCVD risk equivalents included type 2 diabetes, FH, or a 10-year risk of a cardiovascular event of ≥20% as assessed by the Framingham Risk Score for Cardiovascular Disease or equivalent.1
‡ After an initial dose, inclisiran is administered again at 3 months, followed by every 6 months.2
AE, adverse event; ASCVD, atherosclerotic cardiovascular disease; CI, confidence interval; FH, familial hypercholesterolaemia; LDL-C, low-density lipoprotein cholesterol.
References
- Ray KK et al. N Engl J Med 2020;382(16):1507–1519.
- Leqvio® Summary of Product Characteristics. Accessed August 2022 at www.ema.europe.eu
- Ray KK et al. N Engl J Med 2020;382(16):1507–1519 (supplementary appendix).
Abbreviated Prescribing Information
Please refer to Summary of Product Characteristics (SmPC) before prescribing.
Leqvio® (inclisiran) solution for injection in pre-filled syringe
Presentation: Pre-filled syringe containing inclisiran sodium equivalent to 284 mg inclisiran in 1.5 ml solution. Indications: In adults with primary hypercholesterolaemia (heterozygous familial and non-familial) or mixed dyslipidaemia, as an adjunct to diet: • in combination with a statin or statin with other lipid lowering therapies in patients unable to reach LDL-C goals with the maximum tolerated dose of a statin, or • alone or in combination with other lipid lowering therapies in patients who are statin intolerant, or for whom a statin is contraindicated. Dosage and Administration: One single subcutaneous injection: initially, again at 3 months, followed by every 6 months. Inclisiran can be administered immediately after the last dose of a monoclonal antibody PCSK9 inhibitor and is recommended to be administered within 2 weeks of the last dose of a monoclonal antibody PCSK9 inhibitor to maintain LDL-C lowering. No dose adjustment is required in elderly patients, in patients with mild, moderate, severe renal impairment, in patients with end-stage renal disease, or in patients with mild or moderate hepatic impairment. No data available for patients with severe hepatic impairment. The safety and efficacy in patients below 18 years of age not been established. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Warnings/Precautions: Haemodialysis: should not be performed for at least 72 hours after inclisiran dosing. Interactions: Inclisiran is not expected to have clinically significant interactions with other medicinal products. Inclisiran is not a substrate for common drug transporters and, it is not anticipated to be a substrate for cytochrome P450. Inclisiran is not an inhibitor or inducer of cytochrome P450 enzymes or common drug transporters. Fertility, Pregnancy and Lactation: As a precautionary measure, avoid the use of inclisiran during pregnancy. It is unknown whether inclisiran is excreted in human milk. A decision must be made whether to discontinue breast-feeding or to discontinue/abstain from therapy, taking into account the benefit of lactation for the child and the benefit of therapy for the woman. No data on the effect of inclisiran on human fertility are available. Undesirable Effects: Common (≥1/100 to <1/10): adverse reactions at the injection site. The most frequently occurring adverse reactions at the injection site were injection site reaction, injection site pain, injection site erythema, and injection site rash. All of these adverse reactions were mild or moderate in severity, transient and resolved without sequelae. Pack Size(s): Single pack containing one pre-filled syringe. Legal Category: POM. Product (Marketing) Authorisation Number(s): EU/1/20/1494/001. Product (Marketing) Authorisation Holder: Novartis Europharm Limited, Vista Building, Elm Park, Merrion Road, Dublin 4, Ireland. Full prescribing information is available upon request from: Novartis Ireland Limited, Vista Building, Elm Park Business Park, Elm Park, Dublin 4. Tel: 01-2601255 or at www.medicines.ie. Detailed information on this product is also available on the website of the European Medicines Agency http://www.ema.europa.eu. Prescribing Information last revised: December 2020
Reporting suspected adverse reactions of the medicinal product is important to Novartis and the HPRA. It allows continued monitoring of the benefit/risk profile of the medicinal product. All suspected adverse reactions should be reported via HPRA Pharmacovigilance, website www.hpra.ie. Adverse events could also be reported to Novartis preferably via www.report.novartis.com or by email: [email protected] or by calling 01 2080 612.
Reporting of side effects
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk profile of the medicinal product. All suspected adverse reactions should be reported to HPRA Pharmacovigilance, website www.hpra.ie. Adverse events can also be reported to Novartis preferably at www.novartis.com/report, by emailing [email protected] or by calling (01) 2080 612.
![]()
![]()
