Dosing and product information1
PsA, AS and nr-axSpA dosing: a convenient dosing schedule that delivers all the benefits of Cosentyx
Cosentyx, alone or in combination with MTX, is indicated for the treatment of active psoriatic arthritis in adult patients when the response to previous DMARD therapy has been inadequate.
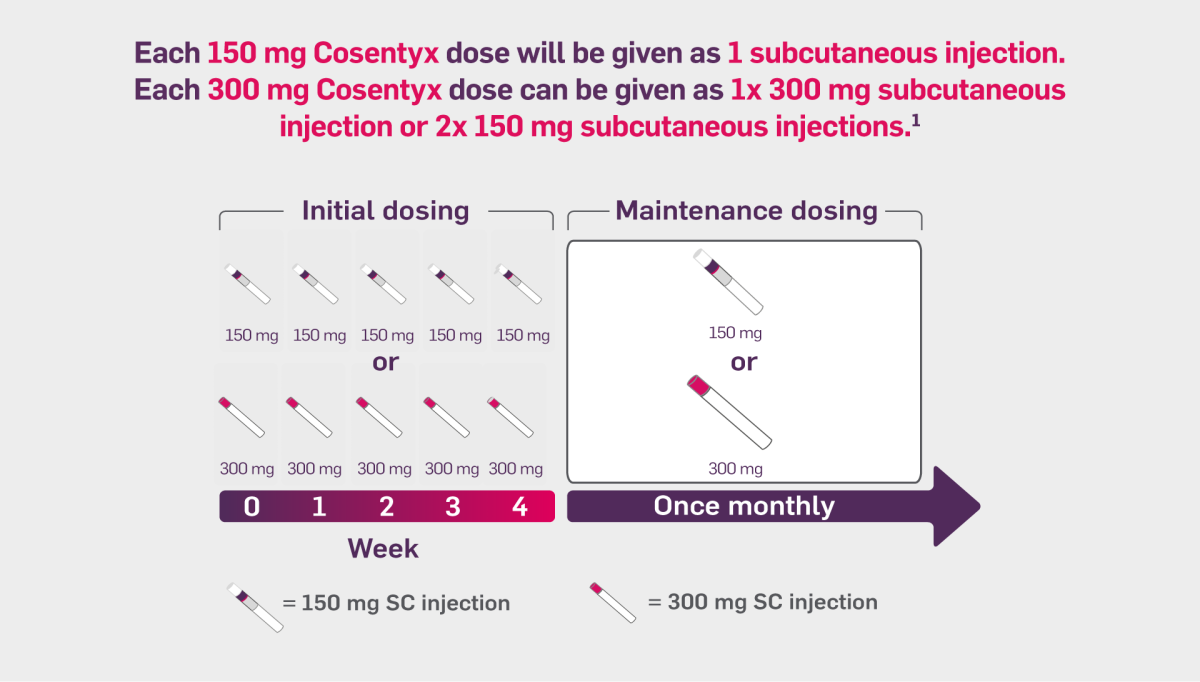
For adult patients with concomitant moderate to severe plaque psoriasis, the recommended dose is 300 mg of secukinumab by subcutaneous injection, with initial dosing at Weeks 0, 1, 2, 3 and 4, followed by monthly maintenance dosing. Based on clinical response, a maintenance dose of 300 mg every 2 weeks may provide additional benefit for patients with a body weight of 90 kg or higher. Each 300 mg dose is given as one subcutaneous injection of 300 mg or as two subcutaneous injections of 150 mg.
For patients who are anti TNFα inadequate responders, the recommended dose is 300 mg by subcutaneous injection with initial dosing at Weeks 0, 1, 2, 3 and 4, followed by monthly maintenance dosing. Each 300 mg dose is given as one subcutaneous injection of 300 mg or as two subcutaneous injections of 150 mg.
For other patients, the recommended dose is 150 mg by subcutaneous injection with initial dosing at Weeks 0, 1, 2, 3 and 4, followed by monthly maintenance dosing. Based on clinical response, the dose can be increased to 300 mg.
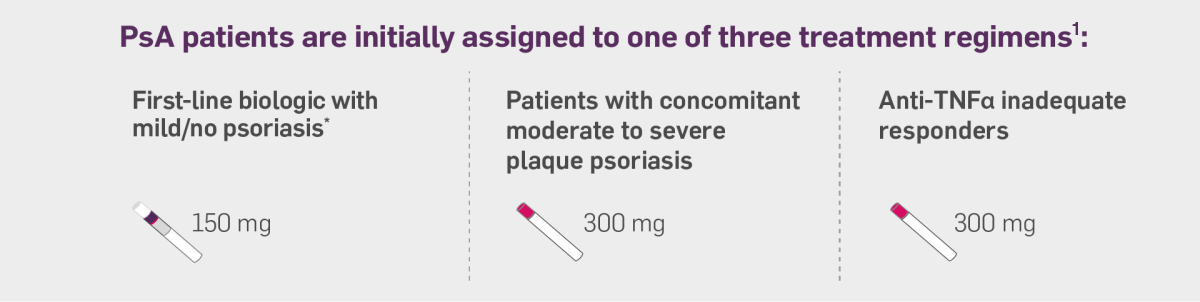
*PsA patients initiated on 150 mg can be increased to 300 mg based on clinical response1.
Please see below the infographics that will guide you on the various Cosentyx dosing regimens dependent on the patient type:
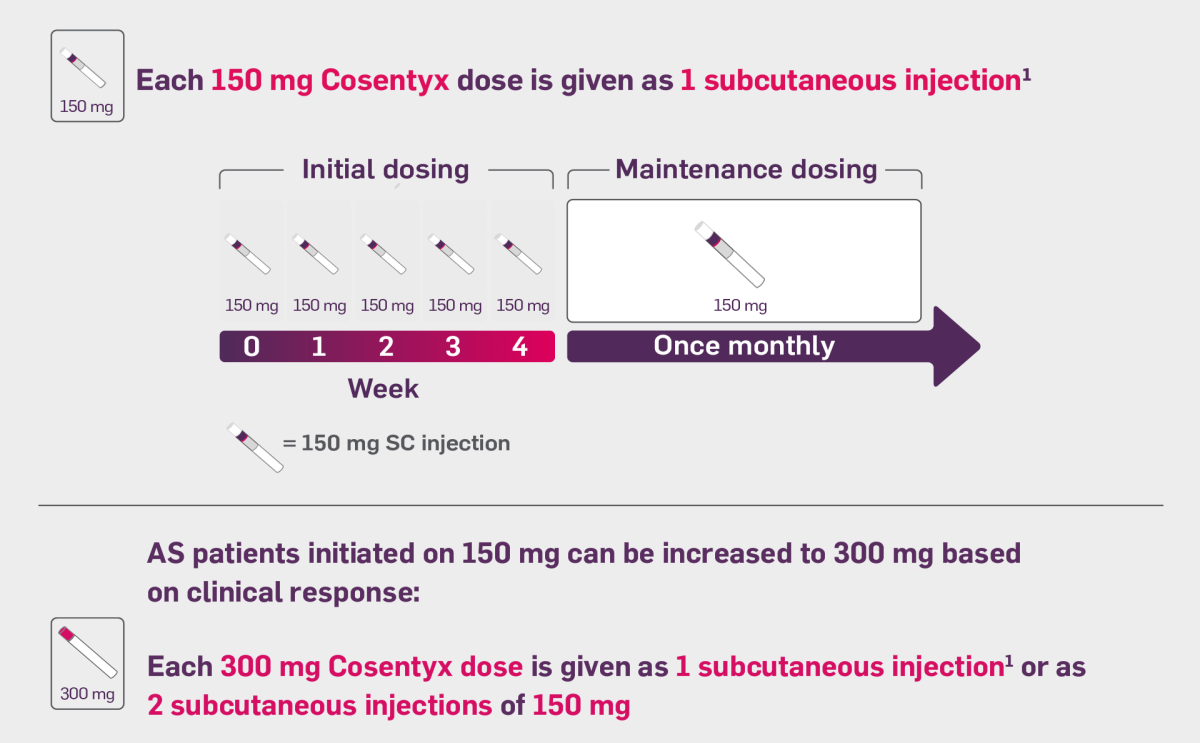
Cosentyx is indicated for the treatment of active ankylosing spondylitis in adults who have responded inadequately to conventional therapy.
For patients with active AS, the recommended dose is 150 mg by subcutaneous injection with initial dosing at Weeks 0, 1, 2, 3 and 4, followed by monthly maintenance dosing. AS patients initiated on 150 mg can be increased to 300 mg based on clinical response.
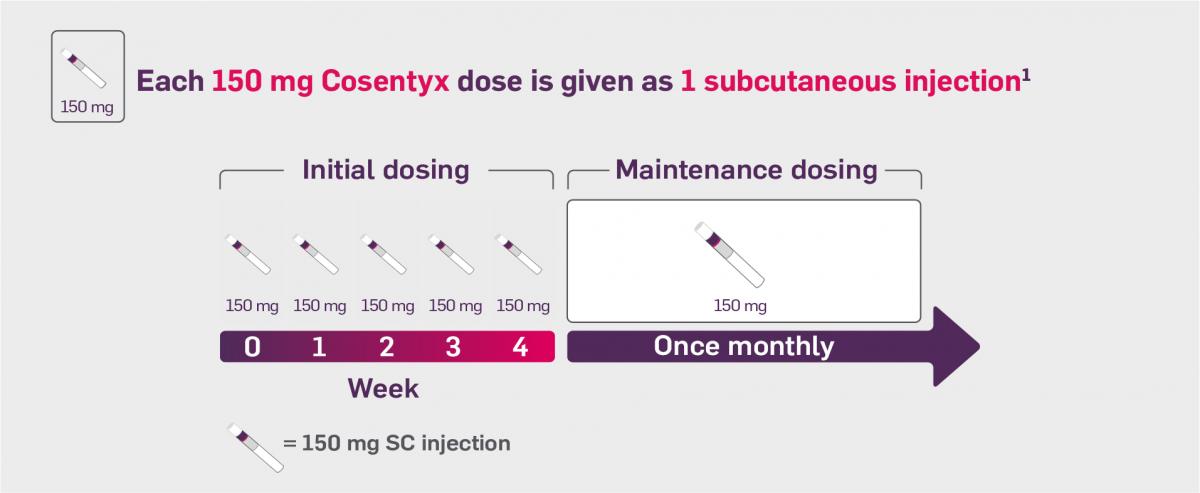
Cosentyx is indicated for the treatment of active non-radiographic axial spondyloarthritis with objective signs of inflammation (CRP and/or MRI evidence) in adults who have responded inadequately to non-steroidal anti-inflammatory drugs.
For patients with active nr-axSpA, the recommended dose is 150 mg by subcutaneous injection with initial dosing at Weeks 0, 1, 2, 3 and 4, followed by monthly maintenance dosing.
- Cosentyx Summary of Product Characteristics, available at www.medicines.ie. Accessed November 2024.
