For full safety information, please refer to the GB and NI Summary of Product Characteristics.1,2
Choose Entresto® wherever your patients are on their chronic HFrEF journey1–12
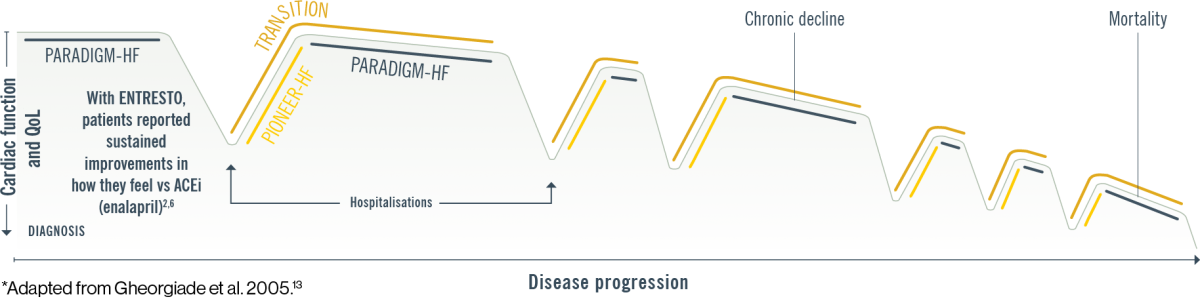
Patients on ENTRESTO feel better, stay out of the hospital, and live longer vs ACEi (enalapril)1–7
Could your chronic symptomatic heart failure patients benefit from ENTRESTO®?†
![]()
Newly diagnosed patients
![]()
For hospitalised patients or patients in the community living with heart failure
There is a wealth of data supporting the use of ENTRESTO in your symptomatic chronic HFrEF patients1,2,4–10
![]()
PARADIGM-HF (N=8,442)
ENTRESTO showed superior efficacy in reducing HF hospitalisations, CV death, and improving QoL, with comparable safety and tolerability vs ACEi (enalapril)3–6
![]()
PIONEER-HF (N=881)
ENTRESTO showed significantly greater reductions in NT-proBNP and exploratory clinical outcomes, with comparable safety vs ACEi (enalapril), when initiated in hospital following haemodynamic stabilisation after an ADHF†‡7
![]()
EVALUATE-HF (N=465)
ENTRESTO showed improvements in reverse cardiac remodelling, with comparable safety vs ACEi (enalapril; the primary endpoint was not met in the EVALUATE-HF trial)§8
![]()
TRANSITION (N=1,002)
ENTRESTO showed comparable safety and tolerability when initiated pre- or post-discharge following haemodynamic stabilisation after an ADHF†9
![]()
PROVE-HF (N=794)
ENTRESTO was associated with reverse cardiac remodelling and improvements in markers of cardiac function. Safety events were comparable to those reported in previous trials10
ENTRESTO is indicated in adult patients for the treatment of symptomatic chronic heart failure with reduced ejection fraction.3
*Schematic representation of the effect of ENTRESTO on the progression of the disease, based on PARADIGM HF3–6 and PIONEER HF7 trials. PARADIGM HF was a multinational, randomised, double-blind trial comparing ENTRESTO to enalapril in 8442 symptomatic (NYHA Class II IV) HFrEF patients (LVEF ≤40%, amended later to ≤35%). For the primary endpoint, composite of CV death or first HF hospitalisation, ENTRESTO® was superior to enalapril (P<0.0001). The median follow-up duration was 27 months.4 A post hoc analysis of PARADIGM HF estimated the long-term treatment effects of ENTRESTO vs enalapril by deriving actuarial estimates of age-specific event rates and expected survival times using data regarding the age at randomisation and the age at the time of an outcome event. Survival analysis was performed using the patients’ age as the time scale (rather than the time since randomisation) to estimate the projected effect of ENTRESTO vs enalapril over the duration of patients’ lifetimes. The effect of treatment on the average duration of event-free survival was estimated by comparing the area under the survival curves.1 PIONEER HF was a prospective, multicentre, double-blind, randomised controlled trial designed to assess the safety, tolerability, and efficacy of in-hospital initiation of ENTRESTO compared with enalapril in 881 adult patients in the United States with HFrEF (EF ≤40% and NT-proBNP ≥1600 pg/mL or BNP ≥400 pg/mL) stabilised during hospitalisation for ADHF. The primary endpoint was time-averaged proportional change in NT-proBNP from baseline through Weeks 4 and 8. An exploratory clinical endpoint was the outcome of a composite of serious clinical events, which included death, rehospitalisation for heart failure, implantation of a left ventricular assist device, and inclusion on the list of patients eligible for heart transplantation.7
† ENTRESTO is indicated in adult patients for treatment of symptomatic chronic heart failure with reduced ejection fraction. ENTRESTO is not indicated for the treatment of acute HF.3 Patients in the PIONEER-HF/TRANSITION trial were required to be haemodynamically stabilised from an ADHF while in hospital.7,9
‡ Primary endpoint: Time-averaged proportional change in NT-proBNP concentration from baseline through Weeks 4 and 8.
§ The primary endpoint was the between-group difference (ENTRESTO vs enalapril) from baseline to Week 12 in aortic characteristic impedance (Zc), a measure of central aortic stiffness.
ACEi, angiotensin converting enzyme inhibitor; ADHF, acute decompensated heart failure; ARB, angiotensin receptor blocker; BNP, brain natriuretic peptide; CaReMe UK, Cardio-Renal-Metabolic Partnership UK; CV, cardiovascular; EF, ejection fraction; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal-pro-brain natriuretic peptide; NYHA, New York Heart Association; QoL, quality of life.
References:
- Claggett B, et al. N Engl J Med 2015;373(23):2289–2290.
- Lewis EF, et al. Circ Heart Fail 2017;10(8):e003430.
- ENTRESTO Summary of Product Characteristics. Accessed May 2024 at www.medicines.ie
- McMurray JJ, et al. N Engl J Med 2014;371(11):993–1004.
- Solomon SD, et al. JACC Heart Fail 2016;4(10):816–822.
- Chandra A, et al. JAMA Cardiol 2018;3(6):498–505.
- Velazquez EJ, et al. N Engl J Med 2019;380(6):539–548.
- Desai AS, et al. JAMA 2019;322(11):1077–1084.
- Wachter R, et al. Eur J Heart Fail 2019;21(8):998–1007.
- Januzzi JL Jr, et al. JAMA 2019;322(11):1085–1095.
- Maddox TM, et al. J Am Coll Cardiol 2021(6);77:772–810.
- McDonagh T, et al. Eur Heart J 2021;42(36):3599-3726.
- Gheorghiade M, et al. Am J Cardiol 2005;96(6A):11G–17G.
- DeVore AD, et al. Initiation of angiotensin-neprilysin inhibition after acute decompensated heart failure: results of the open-label extension of the PIONEER-HF trial. Data presented at American College of Cardiology 68th Annual Scientific Session, March 2019.
- Morrow DA, et al. Circulation 2019;139(19):2285–2288.

