UnoReady® 300mg pen
All the benefits of Cosentyx 300mg now available in ONE 300mg UnoReady® pen1

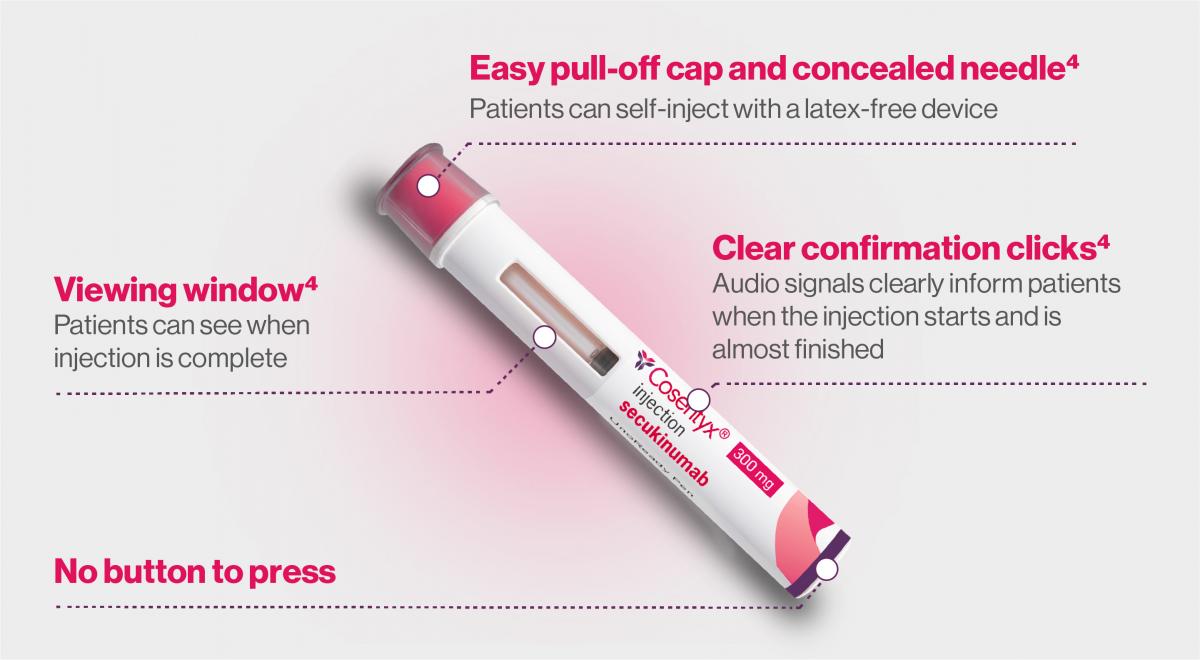
UnoReady® 300mg pen:
Similar administration steps as the SensoReady® 150mg pen, with a few differences.5


The Cosentyx® SensoReady® 150 mg pen will remain available, giving you the option to choose a dose based on your patients’ needs.
If you would like to order a demo-kit or for further information on the UnoReady® 300mg pen, please contact your Novartis Representative.
*Individual patient dosing may vary - please consult the Prescribing Information for full details. Psoriatic Arthritis: Recommended dose is 300mg in patients with concomitant moderate to severe plaque psoriasis or who are anti-TNFα inadequate responders. For other patients, the recommended dose is 150mg. Based on clinical response, the dose can be increased to 300mg. Ankylosing Spondylitis: Recommended dose is 150mg. Based on clinical response, the dose can be increased to 300mg. response. Non-radiographic axial spondyloarthritis: Recommended dose is 150mg.
†N=37, moderate-to-severe plaque psoriasis patients at Week 28.2
‡In a clinical study, adverse events with the preferred term injection-site bruising or injection-site pain due to administration of Cosentyx using the UnoReady® pen over 52 weeks.
DMARD, disease-modifying anti-rheumatic drugs; MTX, methotrexate; NSAIDs, non-steroidal anti-inflammatory drugs; PsA, psoriatic arthritis.
References
- Cosentyx Summary of Product Characteristics. Available at www.medicines.ie. Accessed November 2024.
- Novartis data on file. CAIN457A2325 Data Analysis Report. Satisfaction with self-injection. December 2020.
- Novartis data on file. CAIN457A2325 Data Analysis Report. Injection site reaction rate. December 2020.
- Cosentyx 300mg Patient Leaflet. Available at www.medicines.ie. Accessed March 2023.
- Paul C et al. J Eur Acad Dermatol Venereol. 2015;29(6):1082-1090.