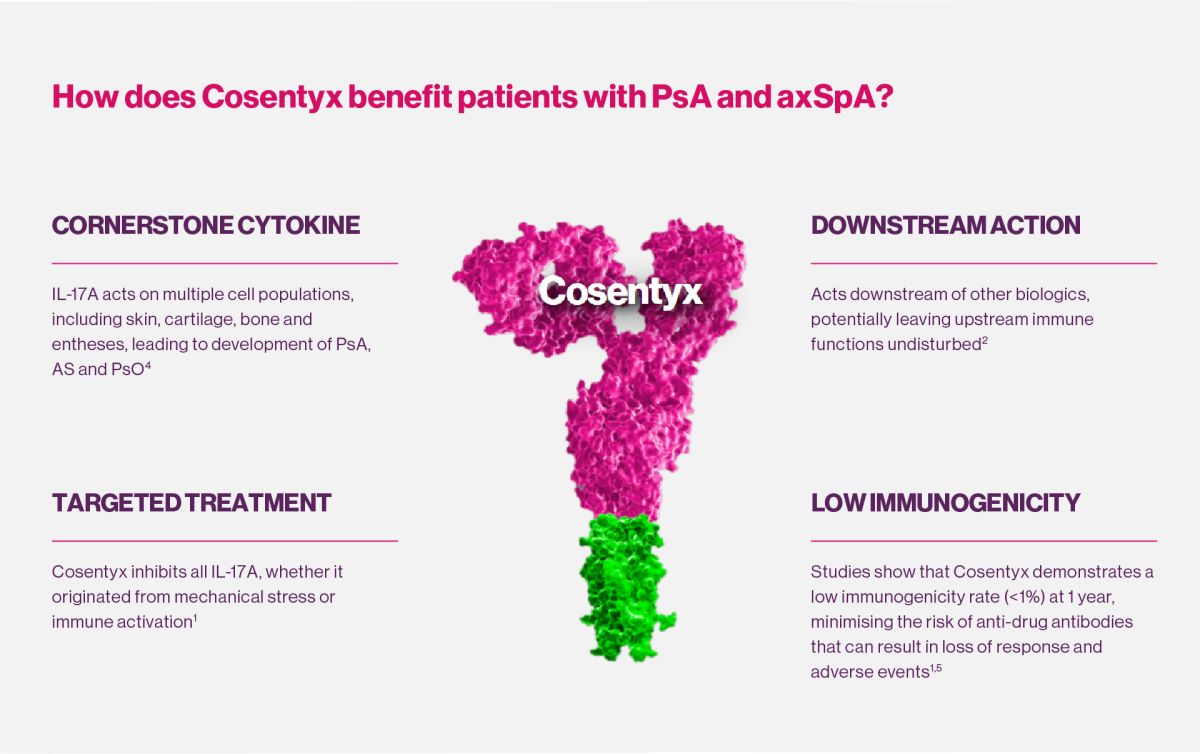
Why target IL-17A?
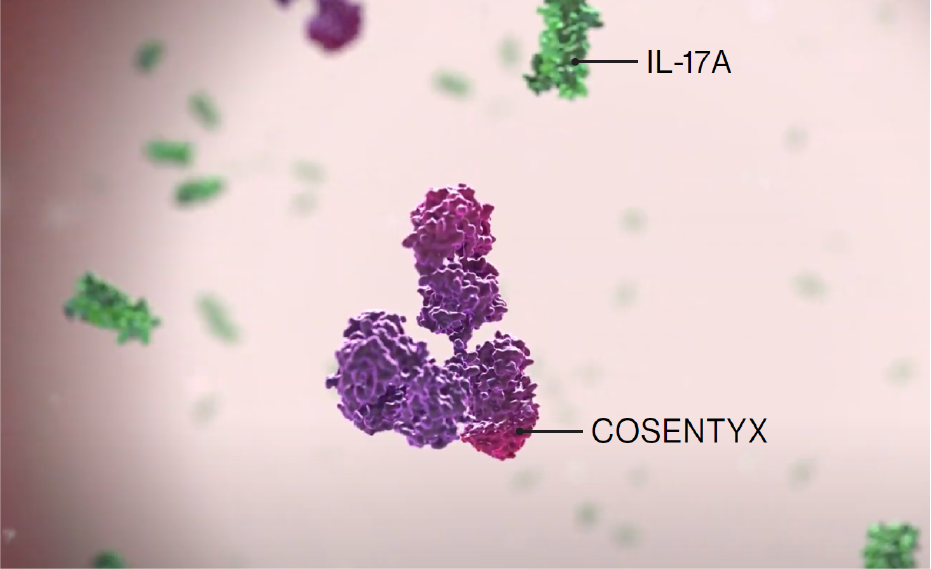
Cosentyx is the first and only fully human targeted IL-17A inhibitor approved for use in PsA and axSpA1
IL-17A is a cornerstone cytokine that plays a critical role at inflammation sites associated with PsA and axSpA indications.2,4
AS, ankylosing spondylitis; axSpA, axial spondyloarthritis; DMARD, disease-modifying antirheumatic drug; IL, interleukin; MRI, magnetic resonance imaging; nr-axSpA, non-radiographic axial spondyloarthritis; NSAID, non-steroidal anti-inflammatory drug; PASI, Psoriasis Area and Severity Index; PsA, psoriatic arthritis; PsO, plaque psoriasis.
- Cosentyx (secukinumab) Summary of Product Characteristics. Available at www.medicines.ie. Accessed November 2024.
- Patel DD et al. Ann Rheum Dis. 20 13;72 (suppl. 2):ii116 -23.
- Kirkham B et al. Immunology. 2013;141:133–42.
- Miossec P and Kolls JK. Nat Rev Drug Discov. 2012;11:763–76.
- Spindeldreher S et al. Dermatol Ther (Heidelb). 2018;8:57–68.