For a trusted response in paediatric immune thrombocytopenia (ITP) patients
Managing ITP in children
Children diagnosed with ITP show a high rate of spontaneous remission* which may occur at any time. Due to this, a 'watch and wait' strategy is often taken with disease management.1,2 However, there are cases where paediatric ITP patients may benefit from treatment, including:
- If they are at higher risk of serious haemorrhage2
- If they are diagnosed at an older age1,2†
- If their health-related quality of life (HRQoL) is compromised and activities are reduced2
For children with chronic or persistent ITP, the International Consensus Report (ICR) encourages the use of thrombopoietin receptor agonists (TPO-RAs). TPO-RAs, such as REVOLADE, are recommended for paediatric patients where alleviating disease will improve HRQoL and/or reduce the risk of bleeding2
REVOLADE could help improve your paediatric patients’ lives through responses that are:3,4
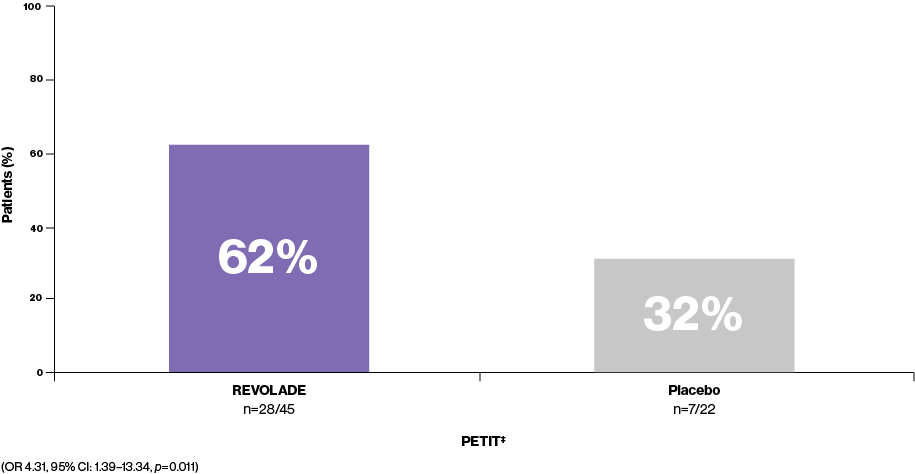
Platelet counts ≥50,000/μL as early as Week 13‡
REVOLADE is nearly twice as effective as placebo at achieving a platelet response (n=28/45 vs. n=7/22, respectively). The majority of paediatric REVOLADE patients (62%, n=28/45) are able to achieve platelet counts ≥50,000/μL at least once during Weeks 1–6 without rescue therapy3
Percentage of patients achieving platelet counts ≥50,000/μL at least once during Weeks 1–6 without rescue therapy3
Platelet counts ≥50,000/μL maintained for ≥6 weeks4§
Regardless of age group, REVOLADE patients are able to achieve and maintain higher plateler counts. 40% (n=25/63) of paediatric REVOLADE patients maintain platelet counts ≥50,000/μL for ≥6 weeks without rescue therapy, compared to 3% (n=1/29) receiving placebo4
Percentage of patients maintaining platelet counts ≥50,000/μL for ≥6 weeks without rescue therapy4
REVOLADE could also offer your ITP patients:3,4
Reduced bleeding symptoms
Patients receiving REVOLADE (27%, n=12/45) had less clinically significant bleeding vs. patients receiving placebo (59%, n=13/22; OR 0.21, 95% CI 0.06–0.72; p=0·013) as well as reduced incidence of bleeding**3
Reduction or discontinuation of concomitant medication
~46% (n=6/13) of REVOLADE patients were able to reduce or discontinue concomitant ITP medication, with 23% (n=3/13) of patients permanently discontinuing all baseline concomitant drugs††3
Choose REVOLADE when your paeditaric patients and carers want a treatment they can trust1–4
CI, confidence interval; ITP, immune thrombocytopenia; ITT, intent to treat; OR, odds ratio; TPO-RA, thrombopoietin receptor agonist.
*The probability of complete remission of chronic ITP was 50% and 76% at 2 and 5 years after diagnosis, respectively.1
†Age at diagnosis had a statistically significant influence of remission rate, the frequency of 50% remission in patients aged <1 year at presentation was 3.8 times that of patients aged >1 year (p=0.02).1
‡The three-part-randomised, multicentre, placebo-controlled PETIT study assessed ecacy and safety of REVOLADE in children aged 1–17 years with persistent and chronic ITP (defined as >6 months duration and platelets counts <30,000/μL who had received at least 1 previous treatment.
Because the study was the first to evaluate REVOLADE use in children, an initial open-label, dose-finding phase was conducted. The double-blind phase and open-label phase of the study recruited additional patients who had not participated in the dose-finding phase.3
§The two-part-randomised, multicentre, placebo-controlled PETIT2 study assessed ecacy and safety of REVOLADE in children aged 1–17 years with chronic ITP (defined as >12 months duration and platelets counts <30,000/μL). Starting doses for REVOLADE were based on results from the open-label, dose-finding phase of the PETIT study.4
**The primary endpoint (proportion of patients achieving a platelet count of 50,000/μL or more at least once from Weeks 1–6 of the randomised phase of the study in the absence of rescue therapy) was compared between treatment groups using a logistic regression model in the randomised phase for the ITT population.
††Of the 13 patients taking drugs for ITP at baseline in the eltrombopag-only phase of the PETIT study.3
References:
-
Kim C, et al. Yonsei Med J. 2016;57:127–131.
-
Provan D, et al. Blood Adv. 2019;3:3780–3817.
-
Bussel J, et al. Lancet Haematol. 2015:315–325.
-
Grainger J, et al. Lancet. 2015;386:1649–1658.