Improving the symptoms that matter to patients
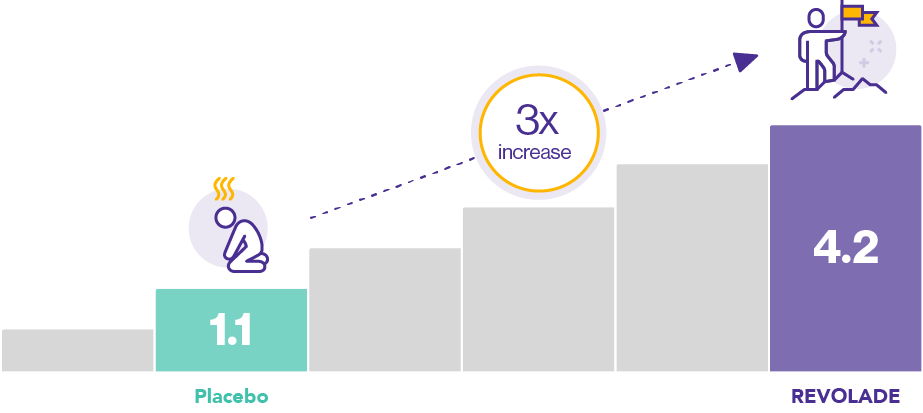
Immune thrombocytopenia (ITP) patients treated with REVOLADE were able to achieve significant improvements in vitality at Week 26, with an almost three-fold increase compared with those receiving placebo [difference 3.1 (4.2 vs. 1.1 respectively), p=0.007, n=135]1*
In the EXTEND study,† at least half (50%–64%) of REVOLADE patients were able to achieve clinically meaningful improvements in health-related quality of life (HRQoL) at least once during treatment2
REVOLADE patients have also been shown to achieve positive mean changes in all HRQoL measures during treatment, with significant improvements seen in measures:2
- Physical health
- Motivation and energy
- Fatigue severity
- Concerns regarding bleeding and bruising
Adjusted mean change in HRQoL score over time compared with baseline
(positive change indicates improvement)²
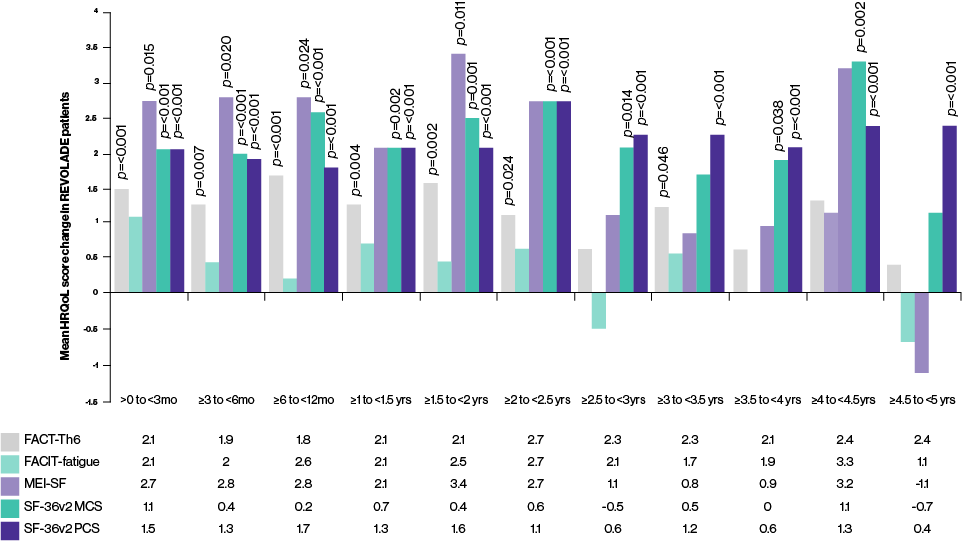
Choose REVOLADE with confidence for a TRUSTED treatment without compromising quality of life1,2
FACT-Th6, Functional Assessment of Cancer Therapy - Thrombocytopenia 6 Item Version; FACIT-fatigue, Functional Assessment of Chronic Illness Therapy - fatigue scale; HRQoL, health-related quality of life; ITP, immune thrombocytopenia; MEI-SF, Motivation and Energy Inventory-Short Form; SF-36v2 MCS, 36-Item Short Form Survey version 2 Mental Component Score; SF-36v2 PCS, 36-Item Short Form Survey version 2 Mental Component Score.
*The phase III, double-blind, placebo-controlled RAISE study evaluated the response of ITP patients (defined as >6 months duration with baseline platelet counts <30,000/µL) to REVOLADE over 6 months of treatment.1
†The phase III, open-label EXTEND study evaluated long-term safety and ecacy of REVOLADE in adults with ITP (defined as >6 months duration with baseline platelet counts <30,000/µL) who have completed a previous REVOLADE study. The study reviewed more than 8 years of continuous treatment; 302 patient were enrolled and 135 (45%) completed the study, 60% were treated for at least 2 years and 35% at least 3 years.2
References:
-
Cheng G, et al. Lancet. 2011;377:393–402.
-
Khelif A, et al. Am J Hematol. 2019;94:200–208.